A periodic table is a table that organises all known elements into groups based on their properties with like elements grouped together in the same vertical column and dissimilar elements separated. This similarity in the properties of every eighth element can be illustrated as follows.
What Trend In Electronegativity Do You See As You Go Across A Period Row On The Periodic Table Socratic
The atomic weights of the elements were used in all previous attempts to classify them.

. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
Its monatomic form H is the most abundant chemical substance in the Universe constituting. Newlands Law of Octaves states that when the elements are arranged in increasing order of atomic mass the periodicity in properties of two elements which have an interval of seven elements in between. Historical Development of Periodic Table.
He observed a trend wherein every eighth element exhibited properties similar to the first. Its monatomic form H is the most abundant chemical substance in the Universe constituting. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
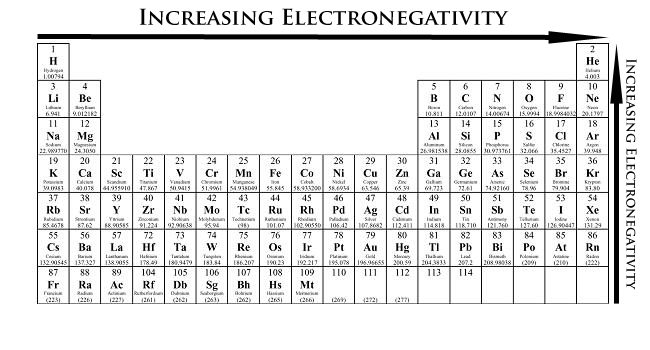
Periodic Trends Chemistry Libretexts

What Is Electronegativity Trends Chart Periodic Table Chemtalk

Electronegativity Definition And Trend

Periodic Trends Made Easy Chemtalk

Periodic Trends Made Easy Chemtalk
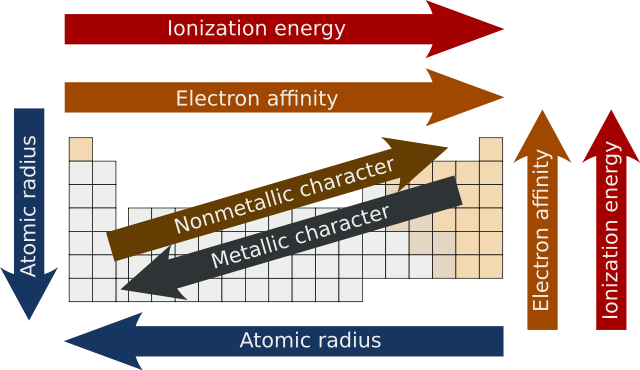
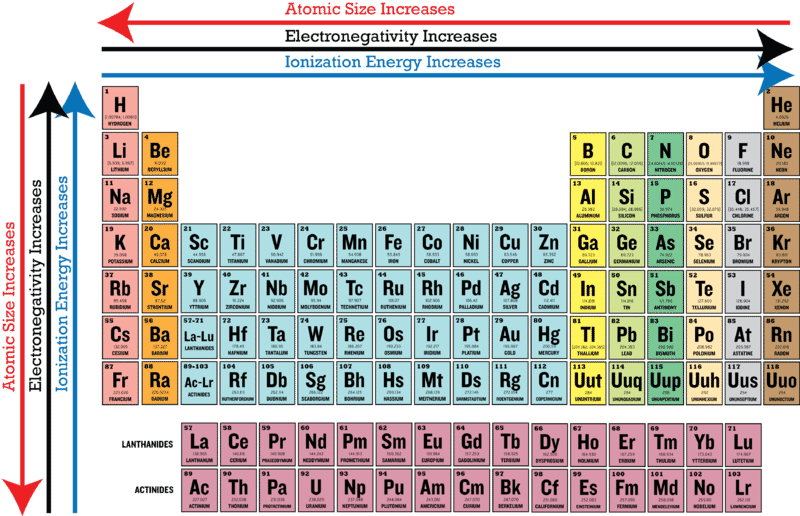
0 comments
Post a Comment